| Intro | | About | | Wiki | | Search traits | | Data explorer | | Literature | | Definitions | | Sources | | Webservices | | Statistics | | Feedback | | Editors | | Log in |
HABs taxon detailsOstreopsis siamensis Johs.Schmidt, 1901
233712 (urn:lsid:marinespecies.org:taxname:233712)
accepted
Species
marine,
Schmidt, J. (1901). Flora of Koh Chang. Contributions to the knowledge of the vegetation in the Gulf of Siam. Peridiniales. <em>Botanisk Tidsskrift.</em> 24: 212-221. [details]
Type locality contained in Ko Chang
type locality contained in Ko Chang [details]
Harmful effect Producer of ostreocin A, B, D and E1, analogues of PLTX. Toxic strains known from Japan (Usami et al. 1995; Ukena et al. 2001),...
Harmful effect Producer of ostreocin A, B, D and E1, analogues of PLTX. Toxic strains known from Japan (Usami et al. 1995; Ukena et al. 2001), Tahiti (Chomérat et al. 2020), South China Sea (Gu et al. 2022) and New Zealand (Rhodes et al. 2002). [details] Identification Two morphological features observed in the material studied by Nugyen-Ngoc et al. (2021) may be of taxonomic significance:...
Identification Two morphological features observed in the material studied by Nugyen-Ngoc et al. (2021) may be of taxonomic significance: the shape of the 2'-plate and the micro-morphology of the pores on the thecal plates. The 2'-plate is long and connects to the 3″-plate, thus separating the 3'- and 2″-plates. Most studies of Ostreopsis species including the type species (Schmidt 1901) are not sufficiently detailed to establish the exact shape of the 2'-plate. Verma et al. (2016a) describing O. rhodesiae regarded a long 2'-plate separating the 3'- and 2″-plates (3'- and 3″-plates in the terminology used by Verma et al. 2016a) as a characteristic feature of O. rhodesiae and O. heptagona, but this particular feature seems to occur also in other species. A long 2'-plate is found in Ostreopsis sp. 6 from French Polynesia (Chomérat et al. 2020b) and in O. cf. ovata (Besada et al. 1982; fig. 4) and in the culture strain CBA-6 (as O. cf. ovata) from Indonesia (Penna et al. 2010, fig. 2c; Parsons et al. 2012, fig. 2), which belong the SE Asian clade of O. cf. ovata (Tawong et al. 2014). Rhodes (2011, fig. 2—identical to Faust et al. 1996, fig. 10) shows two cells of Ostreopsis, misidentified as O. lenticularis, with a long 2'-plate. These cells were collected in Puerto Rico and are morphologically similar to the cells identified here as O. siamensis, O. lenticularis has a short obtuse 2'-plate which does not separate the 3'- and 2″-plates (Chomérat et al. 2019, Nguyen-Ngoc et al. 2022). The thecal pores in O. siamensis are lined by a shallow rim which is also seen in Rhodes (2011; Fig. 2) but not in Faust et al. (1996; fig. 15, misidentified as O. lenticularis), nor in species assigned to O. cf. siamensis, see below. Chomérat et al. (2020b) found a similar type of large thecal pores in Ostreopsis sp. 6 from French Polynesia. However, with the current lack of knowledge of the morphological variability within the Ostreopsis, more studies are needed in order to assess the taxonomic value of these features. [details]
Guiry, M.D. & Guiry, G.M. (2025). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway (taxonomic information republished from AlgaeBase with permission of M.D. Guiry). Ostreopsis siamensis Johs.Schmidt, 1901. Accessed through: Lundholm, N.; Bernard, C.; Churro, C.; Escalera, L.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Mertens, K.; Moestrup, Ø.; Murray, S.; Salas, R.; Tillmann, U.; Zingone, A. (Eds) (2009 onwards) IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae at: https://www.marinespecies.org/HAB/aphia.php?p=taxdetails&id=233712 on 2025-05-04
Lundholm, N.; Bernard, C.; Churro, C.; Escalera, L.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Mertens, K.; Moestrup, Ø.; Murray, S.; Salas, R.; Tillmann, U.; Zingone, A. (Eds) (2009 onwards). IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Ostreopsis siamensis Johs.Schmidt, 1901. Accessed at: https://www.marinespecies.org/hab/aphia.php?p=taxdetails&id=233712 on 2025-05-04
Date action by 2006-07-28 07:22:01Z created Camba Reu, Cibran 2015-06-26 12:00:51Z changed db_admin
original description
Schmidt, J. (1901). Flora of Koh Chang. Contributions to the knowledge of the vegetation in the Gulf of Siam. Peridiniales. <em>Botanisk Tidsskrift.</em> 24: 212-221. [details]
context source (Introduced species) Katsanevakis, S.; Bogucarskis, K.; Gatto, F.; Vandekerkhove, J.; Deriu, I.; Cardoso A.S. (2012). Building the European Alien Species Information Network (EASIN): a novel approach for the exploration of distributed alien species data. <em>BioInvasions Records.</em> 1: 235-245., available online at http://easin.jrc.ec.europa.eu [details] Available for editors basis of record Gómez, F. (2005). A list of free-living dinoflagellate species in the world's oceans. <em>Acta Bot. Croat.</em> 64(1): 129-212. [details] additional source Fukuyo Y. 1981. Taxonomical study on benthic dinoflagellates collected in coral reefs. Bull. Jap. Soc. Sci. Fish. 47: 967-978. [details] additional source Guiry, M.D. & Guiry, G.M. (2025). AlgaeBase. <em>World-wide electronic publication, National University of Ireland, Galway.</em> searched on YYYY-MM-DD., available online at http://www.algaebase.org [details] additional source Tomas, C.R. (Ed.). (1997). Identifying marine phytoplankton. Academic Press: San Diego, CA [etc.] (USA). ISBN 0-12-693018-X. XV, 858 pp., available online at http://www.sciencedirect.com/science/book/9780126930184 [details] additional source Lenoir S., Ten-Hage L., Turquet J., Quod J.-P., Bernard C. & Hennion M.-C. 2004. First evidence of palytoxin analogues from an <i>Ostreopsis mascarenensis</i> (Dinophyceae) benthic bloom in Southwestern Indian Ocean. J. Phycol. 40: 1042-1051. [details] additional source Steidinger, K. A., M. A. Faust, and D. U. Hernández-Becerril. 2009. Dinoflagellates (Dinoflagellata) of the Gulf of Mexico, Pp. 131–154 in Felder, D.L. and D.K. Camp (eds.), Gulf of Mexico–Origins, Waters, and Biota. Biodiversity. Texas A&M Press, College [details] additional source Penna, A., Fraga, S., Battocchi, C., Casabianca, S., Giacobbe, M. G., Riobó, P. & Vernesi, C. 2010. A phylogeographical study of the toxic benthic dinoflagellate genus <i>Ostreopsis</i> Schmidt. Journal of Biogeography 37:830-41. [details] additional source Moestrup, Ø., Akselman, R., Cronberg, G., Elbraechter, M., Fraga, S., Halim, Y., Hansen, G., Hoppenrath, M., Larsen, J., Lundholm, N., Nguyen, L. N., Zingone, A. (Eds) (2009 onwards). IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae., available online at http://www.marinespecies.org/HAB [details] additional source Liu, J.Y. [Ruiyu] (ed.). (2008). Checklist of marine biota of China seas. <em>China Science Press.</em> 1267 pp. (look up in IMIS) [details] Available for editors additional source Chang, F.H.; Charleston, W.A.G.; McKenna, P.B.; Clowes, C.D.; Wilson, G.J.; Broady, P.A. (2012). Phylum Myzozoa: dinoflagellates, perkinsids, ellobiopsids, sporozoans, in: Gordon, D.P. (Ed.) (2012). New Zealand inventory of biodiversity: 3. Kingdoms Bacteria, Protozoa, Chromista, Plantae, Fungi. pp. 175-216. [details] toxicology source Onuma Y., Satake M., Ukena T., Roux J., Chanteau S., Rasolofonirina N., Ratsimaloto M., Naoki H. & Yasumoto T. 1999. Identification of putative palytoxin as the cause of clupeotoxism. Toxicon 37: 55-65. [details] toxicology source Usami N., Satatake M., Ishida S., Inoue A., Kan Y. & Yasumoto T. 1995. Palytoxin analogs from the dinoflagellate <i>Ostreopsis siamensis</i>. J. Am. Chem. Soc. 117: 5389-5390. [details] ecology source Leles, S. G.; Mitra, A.; Flynn, K. J.; Tillmann, U.; Stoecker, D.; Jeong, H. J.; Burkholder, J.; Hansen, P. J.; Caron, D. A.; Glibert, P. M.; Hallegraeff, G.; Raven, J. A.; Sanders, R. W.; Zubkov, M. (2019). Sampling bias misrepresents the biogeographical significance of constitutive mixotrophs across global oceans. <em>Global Ecology and Biogeography.</em> 28(4): 418-428., available online at https://doi.org/10.1111/geb.12853 [details] Available for editors ecology source Mitra, A.; Caron, D. A.; Faure, E.; Flynn, K. J.; Leles, S. G.; Hansen, P. J.; McManus, G. B.; Not, F.; Do Rosario Gomes, H.; Santoferrara, L. F.; Stoecker, D. K.; Tillmann, U. (2023). The Mixoplankton Database (MDB): Diversity of photo‐phago‐trophic plankton in form, function, and distribution across the global ocean. <em>Journal of Eukaryotic Microbiology.</em> 70(4)., available online at https://doi.org/10.1111/jeu.12972 [details] ecology source Faust, M.A. (1998). Mixotrophy in tropical benthic dinoflagellates. In: Reguera, B., Blanco, J., Fernandez, L., Wyatt, T. (Eds.), Harmful Algae. Proceedings of the VIII International Conference on Harmful Algae, Vigo, Spain, 1997, Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO, Paris, France, pp. 390–393. [details]  Present Present  Inaccurate Inaccurate  Introduced: alien Introduced: alien  Containing type locality Containing type locality
From regional or thematic species database
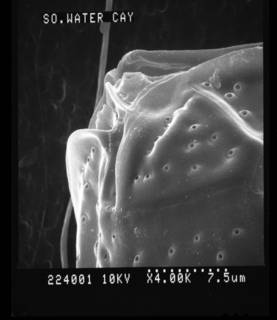
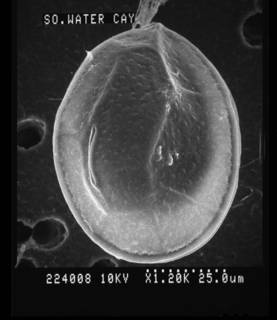
Description Cells collected in natural environments were large in size, but shape and plate patterns were not different from cultured cells. The shape was ovate, broad oval, or tearshaped; dorso-ventral depth (DV) was 91 ± 2 μm (87–95 μm, n = 30), width (W) was 77 ± 3 μm (71–85 μm, n = 30); DV:W ratio was 1.18 ± 0.3 7 (1.0–1.3, n = 30), length (L, apical-antapical dimension) 23–26 μm, (n = 2). The epi- and hypotheca were slightly convex and the cingulum sigmoid due to undulation of cell. The tabulation was APC 4' 6″ 6c 4(?)s 5‴ 2⁗. The Po-plate was strongly eccentric and had a narrow, slitlike apical pore, 13–18 μm long. The 4'-plate was the most conspicuous apical plate; it was elongated, hexagonal, and adjoins the 1'-, 2″-, Po, 1″-, 5″-, 6″-plates, and the sites adjoining the 1″ and 5″ were parallel. The 2'-plate was long and extended to connect to the 3″-plate, thus separating the 3'- and 2″-plates. The 3'-plate was hexagonal adjoining the 2', 4', 3″, 4″, 5″, and the Po-plate for a short distance. The 1‴-plate was small and triangular in shape, while the 2‴–5‴-plates were much larger and about similar in size. The 2⁗-plate was slightly wedged-shaped and located near the center of the hypotheca; the 1⁗ was small. The sulcus was narrow and short, and there were four sulcal plates which were partly covered by the lists of the 5‴- and 1⁗-plates. A small ventral opening (Vo) was present. The thecal plates were smooth and had pores of two size classes: large round pores lined by a shallow elevated rim, 0.39 ± 0.06 μm in diameter (0.26 ± 0.47 μm, n = 30), and smaller pores with a diameter of 0.05 ± 0.03 μm (0.03–0.10, n = 16) were visible only by SEM. [details]Harmful effect Producer of ostreocin A, B, D and E1, analogues of PLTX. Toxic strains known from Japan (Usami et al. 1995; Ukena et al. 2001), Tahiti (Chomérat et al. 2020), South China Sea (Gu et al. 2022) and New Zealand (Rhodes et al. 2002). [details] Identification Two morphological features observed in the material studied by Nugyen-Ngoc et al. (2021) may be of taxonomic significance: the shape of the 2'-plate and the micro-morphology of the pores on the thecal plates. The 2'-plate is long and connects to the 3″-plate, thus separating the 3'- and 2″-plates. Most studies of Ostreopsis species including the type species (Schmidt 1901) are not sufficiently detailed to establish the exact shape of the 2'-plate. Verma et al. (2016a) describing O. rhodesiae regarded a long 2'-plate separating the 3'- and 2″-plates (3'- and 3″-plates in the terminology used by Verma et al. 2016a) as a characteristic feature of O. rhodesiae and O. heptagona, but this particular feature seems to occur also in other species. A long 2'-plate is found in Ostreopsis sp. 6 from French Polynesia (Chomérat et al. 2020b) and in O. cf. ovata (Besada et al. 1982; fig. 4) and in the culture strain CBA-6 (as O. cf. ovata) from Indonesia (Penna et al. 2010, fig. 2c; Parsons et al. 2012, fig. 2), which belong the SE Asian clade of O. cf. ovata (Tawong et al. 2014). Rhodes (2011, fig. 2—identical to Faust et al. 1996, fig. 10) shows two cells of Ostreopsis, misidentified as O. lenticularis, with a long 2'-plate. These cells were collected in Puerto Rico and are morphologically similar to the cells identified here as O. siamensis, O. lenticularis has a short obtuse 2'-plate which does not separate the 3'- and 2″-plates (Chomérat et al. 2019, Nguyen-Ngoc et al. 2022). The thecal pores in O. siamensis are lined by a shallow rim which is also seen in Rhodes (2011; Fig. 2) but not in Faust et al. (1996; fig. 15, misidentified as O. lenticularis), nor in species assigned to O. cf. siamensis, see below. Chomérat et al. (2020b) found a similar type of large thecal pores in Ostreopsis sp. 6 from French Polynesia. However, with the current lack of knowledge of the morphological variability within the Ostreopsis, more studies are needed in order to assess the taxonomic value of these features. [details] |