Scleractinia taxon detailsEchinophyllia Klunzinger, 1879
204799 (urn:lsid:marinespecies.org:taxname:204799)
accepted
Genus
Madrepora aspera Ellis & Solander, 1786 accepted as Echinophyllia aspera (Ellis & Solander, 1786) (type by subsequent designation)
Oxyphyllia Yabe & Eguchi, 1935 · unaccepted > junior subjective synonym
marine,
Klunzinger CB. (1879). Die Korallthiere des Rothen Meeres, 3. Theil: Die Steinkorallen. Zweiter Abschnitt: Die Asteraeaceen und Fungiaceen. 1-100, pls. 1-10. Gutmann, Berlin. [details]
Description 'Polypar zusammengesetzt, blattartig, dünn, unten radiär gerippt, oben mit zerstreuten mehr weniger vorstehenden Kelchen...
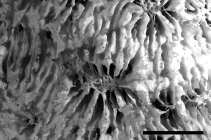
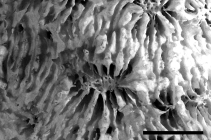
Description 'Polypar zusammengesetzt, blattartig, dünn, unten radiär gerippt, oben mit zerstreuten mehr weniger vorstehenden Kelchen ohne deutliche Mauern, mit wohl entwickelten um die Kelchcentren radiären stark gezähnten Septen; die Kelch durch stark gezähnte subparallele Rippen oder Septa verbunden. Columella deutlich, Unterseite gerippt, mit oder ohne Epithek.' (Klunzinger, 1879: 69) [details] Description Colonies are encrusting, laminar or foliaceous. Calices are round or oval in shape, immersed to tubular, not strongly...
Description Colonies are encrusting, laminar or foliaceous. Calices are round or oval in shape, immersed to tubular, not strongly inclined on the corallum surface. Septa are numerous, columellae are well developed. The coenosteum is pitted at the insertion of new septo-costae. Polyps are extended only at night. (Veron, 1986 <57>) [details]
Hoeksema, B. W.; Cairns, S. (2025). World List of Scleractinia. Echinophyllia Klunzinger, 1879. Accessed at: https://www.marinespecies.org/scleractinia/aphia.php?p=taxdetails&id=204799 on 2025-05-04
Date action by 2006-09-11 06:44:08Z changed Martinez, Olga
original description
Klunzinger CB. (1879). Die Korallthiere des Rothen Meeres, 3. Theil: Die Steinkorallen. Zweiter Abschnitt: Die Asteraeaceen und Fungiaceen. 1-100, pls. 1-10. Gutmann, Berlin. [details]
original description (of Oxyphyllia Yabe & Eguchi, 1935) Yabe H, Eguchi M (1935) Oxyphyllia, a new genus of hexacorals. Proceedings of the Imperial Academy of Japan 11: 376-378. [details] taxonomy source Arrigoni R, Terraneo TI, Galli P, Benzoni F (2014) Lobophylliidae (Cnidaria, Scleractinia) reshuffled: Pervasive . non-monophyly at genus level. Molecular Phylogenetics and Evolution 73: 60-64. [details] basis of record Veron JEN. (1986). Corals of Australia and the Indo-Pacific. <em>Angus & Robertson Publishers.</em> [details] additional source Randall RH. (2003). An annotated checklist of hydrozoan and scleractinian corals collected from Guam and other Mariana Islands. <em>Micronesica.</em> 35-36: 121-137. page(s): 134 [details] additional source Veron JEN, Pichon M. (1980). Scleractinia of Eastern Australia – Part III. Family Agariciidae, Siderastreidae, Fungiidae, Oculinidae, Merulinidae, Mussidae, Pectinidae, Caryophyllidae, Dendrophylliidae. <em>Australian Institute of Marine Science Monograph Series.</em> 4: 1-459. [details] additional source Veron JEN. (2000). Corals of the World. Vol. 1–3. <em>Australian Institute of Marine Science and CRR, Queensland, Australia.</em> [details] additional source Wells JW. (1936). The nomenclature and type species of some genera of recent and fossil corals. <em>American Journal of Science.</em> 31: 97-134., available online at https://ajsonline.org/article/61464 [details] additional source Budd AF, Fukami H, Smith ND, Knowlton N. (2012). Taxonomic classification of the reef coral family Mussidae (Cnidaria: Anthozoa: Scleractinia). <em>Zoological Journal of the Linnean Society.</em> 166 (3): 465-529., available online at https://doi.org/10.1111/j.1096-3642.2012.00855.x [details] additional source Yabe H, Sugiyama T. (1935). Revised list of the reef-corals from the Japanese seas and of the fossil reef corals of the raised reefs and the Ryukyu limestone of Japan. <em>Journal of the Geological Society of Japan.</em> 42: 379-403. page(s): 402 [details] additional source Arrigoni R, Berumen ML, Chen CA, Terraneo TI, Baird AH, Payri C, Benzoni F. (2016). Species delimitation in the reef coral genera Echinophyllia and Oxypora (Scleractinia, Lobophylliidae) with a description of two new species. <em>Molecular Phylogenetics and Evolution.</em> 105: 146-159., available online at https://doi.org/10.1016/j.ympev.2016.08.023 [details] additional source Huang D, Arrigoni R, Benzoni F, Fukami H, Knowlton N, Smith ND, Stolarski J, Chou LM, Budd AF. (2016). Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). <em>Zoological Journal of the Linnean Society.</em> 178(3): 436-481., available online at https://doi.org/10.1111/zoj.12391 [details] additional source Neave, Sheffield Airey. (1939-1996). Nomenclator Zoologicus vol. 1-10 Online. <em>[Online Nomenclator Zoologicus at Checklistbank. Ubio link has gone].</em> , available online at https://www.checklistbank.org/dataset/126539/about [details] additional source Dunn, D. F. (1982). Cnidaria. McGraw-Hill Book Company. New York and other cities., volume 1, pp. 669-706 page(s): 702 [details] redescription Arrigoni, R.; Berumen, M. L.; Stolarski, J.; Terraneo, T. I.; Benzoni, F. (2019). Uncovering hidden coral diversity: a new cryptic lobophylliid scleractinian from the Indian Ocean. <em>Cladistics.</em> 35 (3): 301–328., available online at https://doi.org/10.1111/cla.12346 [details]  Present Present  Inaccurate Inaccurate  Introduced: alien Introduced: alien  Containing type locality Containing type locality
From editor or global species database
Comparison There are no unambiguous apomorphies for Echinophyllia on either the molecular or morphological tree. Three Oxypora species are nested among five Echinophyllia species in subclade F + G (sensu Arrigoni et al., 2014c) on the molecular phylogeny, and these genera are not reciprocally monophyletic on the morphology tree. The clade comprising these three genera is well supported with a bootstrap value of 71 and decay index of 4, and is defined by four synapomorphies: (1) organically united corallites (likelihood of 0.86 based on the Mk1 model); (2) extensive coenosteum (≥ corallite diameter) (likelihood 0.75); (3) columellae ≥ 1/4 of calice width (likelihood 0.92); and (4) loss of epitheca (likelihood 0.84). The sister relationship between Echinophyllia and Oxypora is further supported by the presence of alveoli, which are small pits on the exotheca forming at points of insertion of new septocostae (Chevalier, 1975; Wood, 1983; Veron, 1986; Veron, 2000; Benzoni, 2013). In Oxypora, these pits may penetrate to the undersurface of the colony to form slit-like pores (Vaughan and Wells, 1943; Wells, 1956; Veron and Pichon, 1980; Dai and Horng, 2009). This distinction appears to be merely superficial as they cannot be distinguished based on molecular data or subcorallite morphology. Furthermore, the current Echinophyllia-Oxypora dichotomy belies the peculiar affinities of some constituent species. On the one hand, Echinophyllia echinata (Saville Kent, 1871: 283) and E. tarae Benzoni, 2013: 63, are morphologically similar to Echinomorpha nishihirai—initially placed in Echinophyllia (Veron, 1990)—mainly because they all possess a prominent central (polymorphic) corallite (Benzoni, 2013). On the other hand, this affinity is not supported by both molecular and morphological data. More comprehensive taxonomic and genetic sampling of subclade F + G, especially of Oxypora species, would be necessary to resolve these genera. Mycedium was thought to be a closely-related species to Echinophyllia, and Wells (1954) remarked that the former can only be distinguished by its more inclined orientation of calices on laminar colonies. Detailed examinations of subcorallite morphology by Huang et al. (2014b) and the present study suggest that multiple characters separate them, including tooth base outline, tooth tip orientation, thickening deposits, as well as costa and septum centre clusters. [details]Description 'Polypar zusammengesetzt, blattartig, dünn, unten radiär gerippt, oben mit zerstreuten mehr weniger vorstehenden Kelchen ohne deutliche Mauern, mit wohl entwickelten um die Kelchcentren radiären stark gezähnten Septen; die Kelch durch stark gezähnte subparallele Rippen oder Septa verbunden. Columella deutlich, Unterseite gerippt, mit oder ohne Epithek.' (Klunzinger, 1879: 69) [details] Description Colonies are encrusting, laminar or foliaceous. Calices are round or oval in shape, immersed to tubular, not strongly inclined on the corallum surface. Septa are numerous, columellae are well developed. The coenosteum is pitted at the insertion of new septo-costae. Polyps are extended only at night. (Veron, 1986 <57>) [details] Diagnosis Colonial; laminar. Budding intracalicular; peripheral budding may be present. Corallites may be polymorphic; organically united and lacking distinct calical walls. Monticules absent. Coenosteum spinose; extensive amount (≥ corallite diameter). Calice width medium to large (≥ 4 mm), with low to medium relief (≤ 6 mm). Costosepta mostly confluent. Septa in ≤ three cycles (≤ 36 septa). Free septa irregular. Septa spaced ≤ 11 septa per 5 mm. Costosepta unequal in relative thickness. Columellae trabecular and spongy (> three threads), ≥ 1/4 of calice width, and discontinuous among adjacent corallites with lamellar linkage. Paliform (uniaxial) lobes weakly or moderately developed. Epitheca absent. Endotheca low-moderate (tabular). Tooth base at midcalice elliptical-parallel. Tooth tip forming multiaxial bulb. Tooth height medium (0.3–0.6 mm). Tooth spacing medium (0.3–1.0 mm), with ≤ six teeth per septum. Tooth size equal between wall and septum. Granules scattered on septal face; weak (rounded). Interarea smooth. Walls formed by dominant paratheca and partial septotheca. Thickening deposits with extensive stereome. Costa centre clusters weak; > 0.6 mm between clusters; medial lines strong. Septum centre clusters weak; 0.3–0.5 mm between clusters; medial lines weak. [details] Remark The genus was established by Klunzinger (1879: 69) for the type species Madrepora aspera Ellis and Solander, 1786: 156, as well as Trachypora lacera Verrill, 1864: 53, under the family 'Fungidae' (Klunzinger, 1879: 59). It was thought to be closely related to Halomitra Dana, 1846, Mycedium Milne Edwards and Haime, 1851b, vol. 15: 130, and Echinopora Lamarck, 1816: 252, of which only the first genus is indeed in Fungiidae Dana, 1846: 283. The latter two are nested within Merulinidae Verrill, 1865: 146 (Budd et al., 2012; Huang et al., 2014b). Prior to this, Madrepora aspera was actually grouped with Trachypora lacera Verrill, 1864: 53, in the genus Trachypora Verrill, 1864: 53 (= Oxypora Saville Kent, 1871: 283), which was an attempt to distinguish these species from Halomitra and Echinopora. The association of Echinophyllia, or its junior synonym Oxyphyllia Yabe and Eguchi, 1935a: 377, with the fungiids persisted when Wells (1935) grouped it with Oxypora, Tridacophyllia de Blainville, 1830: 327 (= Pectinia de Blainville, 1825: 201), Mycedium and Physophyllia Duncan, 1884: 118, in Tridacophylliidae Thiel, 1932: 96, which was originally placed in Fungida (see Yabe and Eguchi, 1935b). Furthermore, Oxyphyllia (= Echinophyllia) was placed in Echinoporidae Verrill, 1901: 132, together with Echinopora and Mycedium by Yabe et al. (1936). However, Wells (1935) stated that Physophyllia, and by familial association, Echinophyllia is not in Fungiidae, and furthermore that there are no true synapticulae—a major synapomorphy of Fungiidae—in any of these genera. When Pectiniidae was established by Vaughan and Wells (1943: 196) within Faviida for the five Tridacophylliidae genera above, there was little doubt that Echinophyllia was distinct from fungiids (but see Matthai, 1948), which were characterised by fenestrate septa. Since then, this classification had become convention (e.g. Wells, 1956; Nemenzo, 1959; Chevalier, 1975; Wood, 1983; Veron, 2000) until the challenge posed by molecular data first revealed by Fukami et al. (2004b). Through extensive genetic sampling of Echinophyllia in recent years, consensus that Echinophyllia and Oxypora are sister genera (subclade F + G sensu Arrigoni et al., 2014c) nested within the Lobophylliidae clade (XIX sensu Fukami et al., 2008) is emerging. The remaining three living genera in Pectiniidae are nested within Merulinidae (clade XVII sensu Fukami et al., 2008), and thus Pectiniidae has been synonymised (Budd et al., 2012; see also Huang et al., 2011, 2014b; Arrigoni et al., 2012). The placement of Echinophyllia in Pectiniidae was long held and appeared stable, so the rare note that it resembled an outgroup was particularly prominent. Chevalier (1975) observed that the septal tooth ornamentation is strong and similar to those in 'Mussidae' (= Lobophylliidae), becoming more irregular distally. Our character analysis support this observation, with Echinophyllia displaying similar tooth base and tip outline as other lobophylliids, but with the apex enlarging into a multiaxial bulb by branching into multidirectional tips. Echinophyllia is widely distributed on the reefs of Indo-Pacific, present from the Red Sea and East Africa to as far east as the Marshall Islands in the Northern Hemisphere (Veron, 2000) and Gambier Islands in the Southern Hemisphere (Glynn et al., 2007; Benzoni, 2013). [details] Type designation Subsequent designation by Wells (1936) [details] |