| Home | | Literature | | Log in |
| Diatoms | | Haptophytes | | Dinoflagellates | | Raphidophyceans | | Dictyochophyceans | | Pelagophyceans | | Cyanobacteria | | Greylist | | Harmful non-toxic |
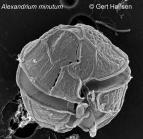
HABs taxon detailsAlexandrium minutum Halim, 1960
109711 (urn:lsid:marinespecies.org:taxname:109711)
accepted
Species
Alexandrium angustitabulatum F.J.R.Taylor, 1995 · unaccepted (synonym, nom. illeg.)
Alexandrium ibericum Balech, 1985 · unaccepted (synonym)
Alexandrium lusitanicum Balech, 1985 · unaccepted (synonym)
Pyrodinium minutum (Halim) Taylor, 1976 · unaccepted (synonym)
marine,
Halim Y. (1960). Alexandrium minutum, n. gen. n. sp. dinoflagellé provocant des “eaux rouges”. <em>Vie et Milieu.</em> 11: 102-105. [details] Available for editors
Type locality contained in Alexandria Harbour
type locality contained in Alexandria Harbour [details]
Harmful effect Producer of STX (Saxitoxin), NeoSTX (Neosaxitoxin) and GTX (Gonyautoxins). Both toxic (i.e. Franco et al. 1994) and...
Harmful effect Producer of STX (Saxitoxin), NeoSTX (Neosaxitoxin) and GTX (Gonyautoxins). Both toxic (i.e. Franco et al. 1994) and non-toxic strains are known. Toxic strains fall into 5 different clusters with different toxin profiles, some of which are grouped geographically while others are widespread. See Lewis et al. (2018) for a review. [details] Identification A. minutum shows morphological similarities to both A. tamutum and A. tamarense. The cell outline of A. tamutum is...
Identification A. minutum shows morphological similarities to both A. tamutum and A. tamarense. The cell outline of A. tamutum is generally rounded or elliptical. This, together with its relatively small size, at first sight is reminiscent of A. minutum, to one of the small-sized species included in the ‘‘A. minutum group,’’ or to the recently described A. camurascutulum. However, A. tamutum cells in their larger size range present a slightly pentagonal shape, thus reflecting the outline of small-sized A. tamarense. The above-mentioned species have distinct size ranges, but they overlap widely; thus, cell size is not a useful character for distinguishing Alexandrium species. The large size of plate 6'' in the precingular series, with a width:height ratio 1, differs from that described for A. minutum and is most similar to the 6'' plate described for A. tamarense. This is the main morphological character that distinguishes our new species from A. minutum. A distinctive morphological feature of this latter species is in fact the very narrow shape and reduced size of the sixth precingular plate. The sulcal posterior plate (Sp) of A. tamutum is of the ‘‘minutum type,’’ viz. roughly rectangular in shape and wider than high, whereas the Sp of A. tamarense is distinctively higher than wide. The small-sized A. camurascutulum also presents a wide 6’’ plate, which, however, is wider than long and has a characteristic hooked shape because of the strongly curved left anterior margin. Moreover, this latter species can be distinguished from A. tamutum and the other species included in the minutum group by a longer sulcal posterior (Sp) plate and by the presence of a large attachment pore on both the sulcal posterior plate and the pore plate. The pore plate of A. tamutum resembles that of A. minutum, with an almost undetectable callus and small marginal pores surrounding the foramen (or comma). Plate 1' has a small ventral pore on its anterior right side. However, whereas in A. minutum the ventral pore is located on the posterior end of the anterior right margin of plate 1', in A. tamutum the pore is situated in the median/upper part of the margin. In all the strains of A. tamutum examined, the connection between the pore plate and plate 1' is clearly visible, whereas in A. minutum this connection is at times hidden by the overgrowth of the margins of plates 2' and 4'. The hypothecal plates of A. tamutum are smooth, whereas those of A. minutum in the type locality and the two strains of A. minutum we examined here also had a smooth hypotheca. Specimens attributed to A. minutum with evident hypothecal ornamentationhave been reported for natural material collected in the Gulf of Naples and Japanese waters. It is not known if the microreticulation is a stable character or if it can be lost in culture conditions, and no molecular data relative to a reticulated morphotype of A. minutum are available to our knowledge. [details] Status Cells identified as A. minutum have been reported globally, covering all continents except Antarctica. Some of these are...
Status Cells identified as A. minutum have been reported globally, covering all continents except Antarctica. Some of these are morphologically somewhat different, and differences in ribosomal gene sequences are also known. It is possible that the reports refer to more than one species or perhaps to subspecies of the same species. [details] Verified sequences Strain CBA-1 (Ignatiades et al. 2007): ITS/5.8S rDNA AJ879163
Verified sequences Strain CBA-1 (Ignatiades et al. 2007): ITS/5.8S rDNA AJ879163 [details]
Guiry, M.D. & Guiry, G.M. (2024). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway (taxonomic information republished from AlgaeBase with permission of M.D. Guiry). Alexandrium minutum Halim, 1960. Accessed through: Lundholm, N.; Churro, C.; Escalera, L.; Fraga, S.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Mertens, K.; Moestrup, Ø.; Murray, S.; Tillmann, U.; Zingone, A. (Eds) (2009 onwards) IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae at: https://www.marinespecies.org/hab/aphia.php?p=taxdetails&id=109711 on 2024-04-19
Lundholm, N.; Churro, C.; Escalera, L.; Fraga, S.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Mertens, K.; Moestrup, Ø.; Murray, S.; Tillmann, U.; Zingone, A. (Eds) (2009 onwards). IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Alexandrium minutum Halim, 1960. Accessed at: https://www.marinespecies.org/hab/aphia.php?p=taxdetails&id=109711 on 2024-04-19
Date action by
original description
Halim Y. (1960). Alexandrium minutum, n. gen. n. sp. dinoflagellé provocant des “eaux rouges”. <em>Vie et Milieu.</em> 11: 102-105. [details] Available for editors
basis of record Guiry, M.D. & Guiry, G.M. (2023). AlgaeBase. <em>World-wide electronic publication, National University of Ireland, Galway.</em> searched on YYYY-MM-DD., available online at http://www.algaebase.org [details] additional source Tomas, C.R. (Ed.). (1997). Identifying marine phytoplankton. Academic Press: San Diego, CA [etc.] (USA). ISBN 0-12-693018-X. XV, 858 pp., available online at http://www.sciencedirect.com/science/book/9780126930184 [details] additional source Ignatiades, L.; Gotsis-Skretas, O.; Metaxatos, A. (2007). Field and culture studies on the ecophysiology of the toxic dinoflagellate <i>Alexandrium minutum</i> (Halim) present in Greek coastal waters. <em>Harmful Algae.</em> 6(2): 153-165., available online at https://doi.org/10.1016/j.hal.2006.04.002 [details] additional source Hallegraeff G.M., Steffensen D.A. & Wetherbee R. 1988. Three estuarine Australian dinoflagellates that can produce paralytic shellfish toxins. J. Plankton Res. 10: 533-541. [details] additional source Balech, E. 1995. The Genus <i>Alexandrium</i> Halim (Dinoflagellata). Sherkin Island Marine Station, Sherkin Island, Co. Cork, Ireland, 151. [details] Available for editors additional source Moestrup, Ø., Akselman, R., Cronberg, G., Elbraechter, M., Fraga, S., Halim, Y., Hansen, G., Hoppenrath, M., Larsen, J., Lundholm, N., Nguyen, L. N., Zingone, A. (Eds) (2009 onwards). IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae., available online at http://www.marinespecies.org/HAB [details] additional source Chang, F.H.; Charleston, W.A.G.; McKenna, P.B.; Clowes, C.D.; Wilson, G.J.; Broady, P.A. (2012). Phylum Myzozoa: dinoflagellates, perkinsids, ellobiopsids, sporozoans, in: Gordon, D.P. (Ed.) (2012). New Zealand inventory of biodiversity: 3. Kingdoms Bacteria, Protozoa, Chromista, Plantae, Fungi. pp. 175-216. [details] redescription Balech, E. (1989). Redescription of Alexandrium minutum Halim (Dinophyceae) type species of the genus Alexandrium. <em>Phycologia.</em> 28(2), 206–211. [details] Available for editors toxicology source Oshima Y., Hirota M., Yasumoto T., Hallegraeff G.M., Blackburn S.I. & Steffensen D.A. 1989. Production of paralytic shellfish toxins by the dinoflagellate <i>Alexandrium minutum</i> Halim from Australia. Bull. Jap. Soc. Sci. Fish. 55: 925. [details] toxicology source Franco J.M., Fernandez P. & Reguera B. 1994. Toxin profiles of natural population and cultures of <i>Alexandrium minutum</i> Halim from Galician (Spain) coastal waters. J. Appl. Phycol. 6: 275-279.<br><br> [details] toxicology source Chang F.H., Anderson D.M., Kulis D.M. & Till D.G. 1997. Toxin production of <i>Alexandrium minutum</i> (Dinophyceae) from the Bay of Plenty, New Zealand. Toxicon 35: 393-409. [details] ecology source Leles, S. G.; Mitra, A.; Flynn, K. J.; Tillmann, U.; Stoecker, D.; Jeong, H. J.; Burkholder, J.; Hansen, P. J.; Caron, D. A.; Glibert, P. M.; Hallegraeff, G.; Raven, J. A.; Sanders, R. W.; Zubkov, M. (2019). Sampling bias misrepresents the biogeographical significance of constitutive mixotrophs across global oceans. <em>Global Ecology and Biogeography.</em> 28(4): 418-428., available online at https://doi.org/10.1111/geb.12853 [details] Available for editors ecology source Mitra, A.; Caron, D. A.; Faure, E.; Flynn, K. J.; Leles, S. G.; Hansen, P. J.; McManus, G. B.; Not, F.; Do Rosario Gomes, H.; Santoferrara, L. F.; Stoecker, D. K.; Tillmann, U. (2023). The Mixoplankton Database (MDB): Diversity of photo‐phago‐trophic plankton in form, function, and distribution across the global ocean. <em>Journal of Eukaryotic Microbiology.</em> 70(4)., available online at https://doi.org/10.1111/jeu.12972 [details] ecology source Jeong, H.; Park, J.; Nho, J.; Park, M.; Ha, J.; Seong, K.; Jeng, C.; Seong, C.; Lee, K.; Yih, W. (2005). Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. <em>Aquatic Microbial Ecology.</em> 41: 131-143., available online at https://doi.org/10.3354/ame041131 [details]  Present Present  Inaccurate Inaccurate  Introduced: alien Introduced: alien  Containing type locality Containing type locality
From regional or thematic species database
Description Cell is small, oval to elliptical in ventral view, and somewhat dorsoventrally flattened. Cingulum is well-excavated, without lists, and descending (usually 1 but sometimes 4). Hypotheca is hemi-elliptical, sometimes somewhat antapically flattened, and scarcely sloping. PO is oval, somewhat irregular, more or less concave to the right, and rather narrow. Callus is very reduced. 1' is narrow, contacting the PO directly or, more often, indirectly by a thread-like prolongation that can be rather long and not easily observed unless the theca is dissected. Ventral pore is small and always very close to the posterior extreme of the anterior right margin. S.a. is approximately as long as it is wide with a straight or aimost straight anterior margin and a slightly deep posterior sinus. S.s.a. is very narrow and rhomboidal. S.s.p. is almost always rather short. S.d.p. has an almost horizontai anterior margin and a convex and oblique posterior margin that is more often subdivided into two almosr straight portions with opposite obliquity. S.d.a. is trianguiar and narrow. Accessory sulcal plates are very small. S.p. is almost always symmetrical. Theca frequently has an irregular areolation that is barely visible, diffuse, and seen mainly in the 1' and even more in the S.p. However, thecae from some locations have an incipient reticulation in the hypotheca. Dimensions: L 15.5-29, the majority 21-26. A equals L, sometimes a little larger but, more often, somewhat smaller. Trd is always smaller than L and 4 to 6 μm less than A. Halim noted L 16-23.2, A 13-20.3 (possibly he was referring to the transdiameter). [details]Harmful effect Producer of STX (Saxitoxin), NeoSTX (Neosaxitoxin) and GTX (Gonyautoxins). Both toxic (i.e. Franco et al. 1994) and non-toxic strains are known. Toxic strains fall into 5 different clusters with different toxin profiles, some of which are grouped geographically while others are widespread. See Lewis et al. (2018) for a review. [details] Identification A. minutum shows morphological similarities to both A. tamutum and A. tamarense. The cell outline of A. tamutum is generally rounded or elliptical. This, together with its relatively small size, at first sight is reminiscent of A. minutum, to one of the small-sized species included in the ‘‘A. minutum group,’’ or to the recently described A. camurascutulum. However, A. tamutum cells in their larger size range present a slightly pentagonal shape, thus reflecting the outline of small-sized A. tamarense. The above-mentioned species have distinct size ranges, but they overlap widely; thus, cell size is not a useful character for distinguishing Alexandrium species. The large size of plate 6'' in the precingular series, with a width:height ratio 1, differs from that described for A. minutum and is most similar to the 6'' plate described for A. tamarense. This is the main morphological character that distinguishes our new species from A. minutum. A distinctive morphological feature of this latter species is in fact the very narrow shape and reduced size of the sixth precingular plate. The sulcal posterior plate (Sp) of A. tamutum is of the ‘‘minutum type,’’ viz. roughly rectangular in shape and wider than high, whereas the Sp of A. tamarense is distinctively higher than wide. The small-sized A. camurascutulum also presents a wide 6’’ plate, which, however, is wider than long and has a characteristic hooked shape because of the strongly curved left anterior margin. Moreover, this latter species can be distinguished from A. tamutum and the other species included in the minutum group by a longer sulcal posterior (Sp) plate and by the presence of a large attachment pore on both the sulcal posterior plate and the pore plate. The pore plate of A. tamutum resembles that of A. minutum, with an almost undetectable callus and small marginal pores surrounding the foramen (or comma). Plate 1' has a small ventral pore on its anterior right side. However, whereas in A. minutum the ventral pore is located on the posterior end of the anterior right margin of plate 1', in A. tamutum the pore is situated in the median/upper part of the margin. In all the strains of A. tamutum examined, the connection between the pore plate and plate 1' is clearly visible, whereas in A. minutum this connection is at times hidden by the overgrowth of the margins of plates 2' and 4'. The hypothecal plates of A. tamutum are smooth, whereas those of A. minutum in the type locality and the two strains of A. minutum we examined here also had a smooth hypotheca. Specimens attributed to A. minutum with evident hypothecal ornamentationhave been reported for natural material collected in the Gulf of Naples and Japanese waters. It is not known if the microreticulation is a stable character or if it can be lost in culture conditions, and no molecular data relative to a reticulated morphotype of A. minutum are available to our knowledge. [details] Status Cells identified as A. minutum have been reported globally, covering all continents except Antarctica. Some of these are morphologically somewhat different, and differences in ribosomal gene sequences are also known. It is possible that the reports refer to more than one species or perhaps to subspecies of the same species. [details] Verified sequences Strain CBA-1 (Ignatiades et al. 2007): ITS/5.8S rDNA AJ879163 [details]
Published in AlgaeBase
 Published in AlgaeBase  (from synonym Alexandrium ibericum Balech, 1985) (from synonym Alexandrium ibericum Balech, 1985)Published in AlgaeBase  (from synonym Alexandrium lusitanicum Balech, 1985) (from synonym Alexandrium lusitanicum Balech, 1985)Published in AlgaeBase  (from synonym Alexandrium angustitabulatum F.J.R.Taylor, 1995) (from synonym Alexandrium angustitabulatum F.J.R.Taylor, 1995)Published in AlgaeBase  (from synonym Pyrodinium minutum (Halim) Taylor, 1976) (from synonym Pyrodinium minutum (Halim) Taylor, 1976)To Barcode of Life (1 barcode) (from synonym Alexandrium lusitanicum Balech, 1985) To Barcode of Life (6 barcodes) To Biodiversity Heritage Library (1 publication) (from synonym Alexandrium ibericum Balech, 1985) To Biodiversity Heritage Library (2 publications) (from synonym Alexandrium lusitanicum Balech, 1985) To Biodiversity Heritage Library (7 publications) To Dyntaxa To Dyntaxa (from synonym Alexandrium angustitabulatum F.J.R.Taylor, 1995) To European Nucleotide Archive (ENA) (from synonym Alexandrium lusitanicum Balech, 1985) To European Nucleotide Archive (ENA) To GenBank (29 nucleotides; 0 proteins) (from synonym Alexandrium lusitanicum Balech, 1985) To GenBank (87072 nucleotides; 17093 proteins) To Global Invasive Species Database (GISD) To Information system on Aquatic Non-Indigenous and Cryptogenic Species (AquaNIS) To PESI To PESI (from synonym Alexandrium angustitabulatum F.J.R.Taylor, 1995) To ITIS |